Abstract
ADCs combine the high specificity of monoclonal antibody drugs with the high activity of small molecule cytotoxic drugs to improve the targeting of oncology drugs. Compared with traditional fully or partially humanized antibodies or antibody fragments, ADCs can release highly active cytotoxins in tumor tissues. Compared with the fusion protein, it has higher tolerance or lower side effects.
ADC drugs have accurate identification of targets and non-cancer cells that are not affected, greatly improving drug efficacy and reducing toxic side effects, and have attracted the attention of personnel in the field of medical research and development.
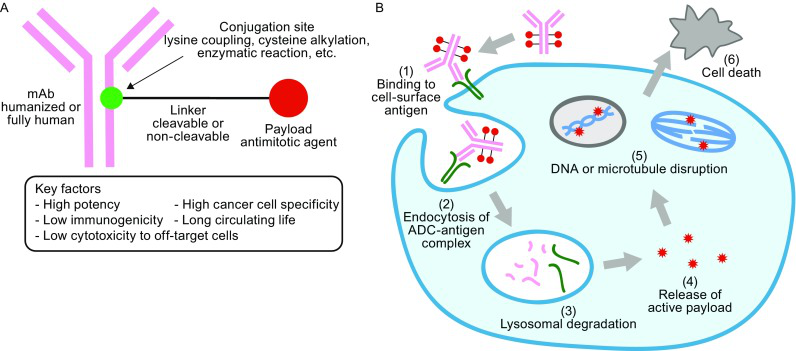
ADCs drugs are composed of recombinant antibodies, chemical drugs, and linkers, and involve four aspects: drug target screening, recombinant antibody preparation, connector technology development, and highly cytotoxic compounds optimization. There must no mistakes in any part of the experimental process because this will affect the safety and effectiveness.
The related technical characteristics of ADCs drugs
ADCs drug targets
The principle of ADCs drug target selection is to specifically express or overexpress antigens in tumor cells to ensure the targeting of ADCs drugs in the body.
Antibodies used in ADCs drugs
After highly cytotoxic chemical drugs are connected to antibodies, they can accurately target cells. The role of antibodies in ADCs drugs is to precisely “guide”.
ADCs drug linker
A linker realizes the connection between the antibody and the chemical drug. The ADCs drug enters the target site must ensure the effective release for chemical drug, which directly affects its pharmacokinetics.
Chemical drugs used by ADCs
The use of main chemical drugs is tubulin inhibitors (natamycin), alkylating agents, and DNA minor groove inhibitors (enediyne antibiotics). Compared with traditional chemotherapy drugs, it has a stronger killing effect on cancer cells.
Pharmacokinetic
The complex structure and mechanism of action make ADC pharmacokinetic research more challenging. ADC is mainly administered intravenously. After the drug enters the body, it can enter cells through specific and non-specific pathways.
These reagents can deliver highly cytotoxic payloads directly to tumor cells, so they can be used to achieve high lethality to target cancer cells without harming healthy cells. Simply says using the specific binding of monoclonal antibodies and antigens to achieve targeted therapy of cancer cells. Antibody-drug conjugates can deliver inactivated cytotoxins to specific cancer cells, then preventing tumor cells from dividing, and kill the cells.
Can an “antibody-chemical drug coupling agent” be directly constructed to use the specific binding ability of the antibody to target cells to deliver highly cytotoxic chemical drugs to achieve effective killing of cancerous cells? The clinical needs have posed new challenges for the pharmaceutical industry to develop anti-cancer drugs.
In the concept of drug design, antibodies become biological missiles for the delivery of chemical drugs at designated locations, and chemical drugs are warheads with lethal effects for biological missiles. They will be able to use antibody drugs and chemical drugs advantages.
Conclusion
To date, nine antibody-drug conjugates have received market approval and nearly 100 investigational ADCs are currently in pre-clinical and clinical trials.
Despite decades of research and development history, ADC still has huge room for improvement. In addition. With the advantages of clear targets, mature technology, and good selectivity, the research of antibody-drug conjugates will be a research hot spot in the field of anti-cancer.
Reference
[1]https://www.adcreview.com/the-review/antibody-drug-conjugates/what-are-antibody-drug-conjugates/
[2]http://blog.sciencenet.cn/blog-454912-732997.html
Related Reading: