Glutamine Metabolism and Cancer Therapy: A Comprehensive Guide
Glutamine metabolism plays a key role in cancer growth, energy production, and biosynthesis. Since Hans Krebs first described the tricarboxylic acid (TCA) cycle in 1935, research has highlighted glutamine’s importance in both normal and cancer cells. Today, targeting glutamine pathways in cancer therapy shows promising potential. This guide explains glutamine’s impact, its role in autophagy and ROS, strategies for targeting it, and considerations for future treatments.
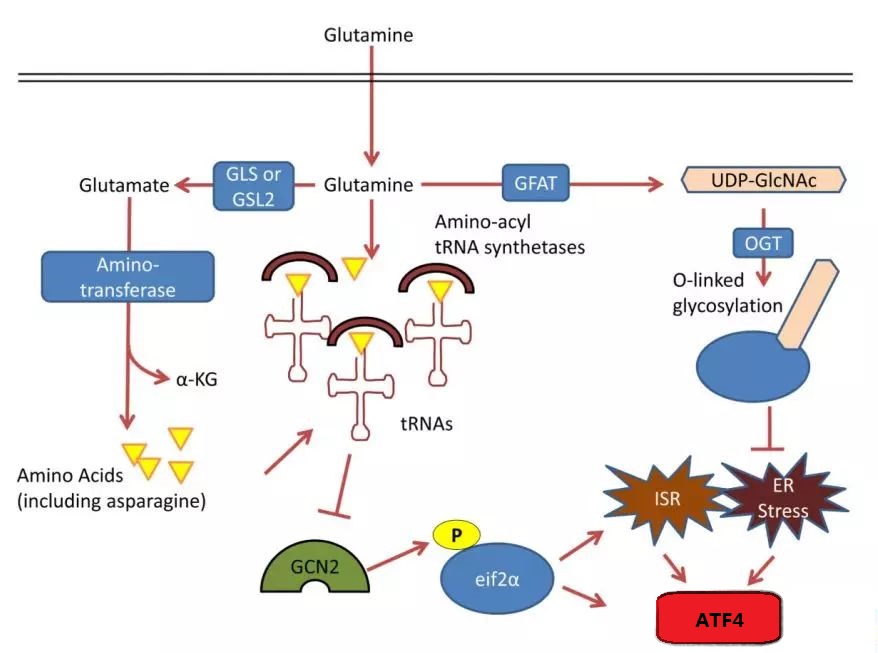
Synthesis of glutamine in nucleic acids, lipids, and proteins
1. Glutamine Metabolism in Cancer Cells
Cancer cells rely heavily on glutamine:
- Energy Production: Glutamine fuels the TCA cycle, converting into α-ketoglutarate to supply energy.
- Biosynthesis: It provides carbon and nitrogen for building nucleotides, lipids, and proteins.
- Metabolic Flexibility: When nutrients are scarce, cancer cells switch to using glutamine as an energy source.
Cancer cells use several strategies to acquire glutamine:
- High Uptake: They absorb glutamine through transporters like SLC1A5.
- Nutrient Scavenging: In stressful conditions, cells break down internal components or engulf proteins to obtain glutamine.
2. Glutamine’s Role in Biosynthesis
-
Nucleic Acid Synthesis
- Glutamine supplies nitrogen for making purines and pyrimidines. Without it, cancer cells may stop dividing.
- It activates the mTOR pathway, stimulating enzymes involved in nucleotide production.
-
Lipid Synthesis
- Glutamine-derived α-ketoglutarate converts to acetyl-CoA, which supports lipid formation.
-
Protein Synthesis
- Glutamine contributes nitrogen for amino acids and helps with protein folding. Low levels can cause protein stress.
3. Glutamine Metabolism Pathways
-
TCA Cycle and IDH2
- In the TCA cycle, glutamine-derived α-ketoglutarate converts to isocitrate and citrate. The enzyme IDH2 aids in this process, fueling energy production.
- Mutations in IDH2 can disrupt glutamine metabolism, affecting cancer growth.
-
Glutathione Pathway
- Glutamine metabolism produces glutamate, a precursor for glutathione. This antioxidant neutralizes harmful reactive oxygen species (ROS).
- NADPH, generated through glutamine metabolism, supports glutathione regeneration, helping keep ROS levels in check.
4. Glutamine and Autophagy in Cancer Research
Glutamine plays a significant role in autophagy, a process where cells break down their own components for nutrients:
- Promoting Cancer Survival: When nutrients run low, glutamine supports autophagy, allowing cancer cells to sustain themselves.
- Dual Role in Tumor Growth: Autophagy can both help and hinder tumor growth. While it reduces oxidative stress and prevents chromosomal instability, it also helps cancer cells survive under stress.
- Interacting with Stress Pathways: Glutamine influences autophagy through pathways like mTOR and GCN2, promoting nutrient acquisition and managing cell stress.
5. Glutamine and ROS (Reactive Oxygen Species) in Cancer Research
Glutamine and ROS
Cancer cells need to control ROS levels to avoid damage:
- Moderate ROS Levels: Low to moderate levels promote cell growth by signaling for cell division.
- High ROS Levels: Too much ROS can damage cells, leading to cell death. Cancer cells need to carefully balance ROS to avoid harm.
- Regulating ROS with Glutamine: Through metabolism, glutamine generates glutathione and NADPH. Both are crucial for neutralizing ROS and protecting cells from damage.
- Influencing the Tumor Microenvironment: Glutamine-derived metabolites help control ROS in the tumor microenvironment, aiding cancer cell adaptation and survival.
6. Glutamine and T Cells
Glutamine isn’t only important for cancer cells. It also fuels immune cells, especially T cells:
- Early Activation: During activation, T cells need glutamine to grow and divide quickly. It provides energy and essential building blocks.
- T Cell Differentiation: Glutamine affects how CD4+ T cells develop into different subtypes, influencing immune responses.
7. Strategies for Targeting Glutamine Metabolism
Disrupting glutamine metabolism can weaken cancer cells:
- Glutaminase Inhibitors (e.g., CB-839): These drugs block glutaminase, preventing the conversion of glutamine to glutamate.
- Transporter Inhibitors: Targeting glutamine transporters like SLC1A5 reduces glutamine uptake, depriving cells of nutrients.
- Combination Treatments: Pairing glutamine inhibitors with chemotherapy, immunotherapy, or IDH2 inhibitors boosts effectiveness.
8. Key Considerations for Glutamine-Targeted Therapies
Clinical of glutaminase inhibitors
-
Metabolic Flexibility
- Cancer cells adapt to nutrient changes by switching metabolic pathways. Understanding these shifts can uncover new treatments.
-
Tumor Microenvironment
- Factors like hypoxia and acidity affect how cells use glutamine. Adding therapies that modify the tumor environment may enhance outcomes.
-
Glutamine Addiction
- Some tumors rely heavily on glutamine. Combining glutamine-targeted treatments with oncogene inhibitors may create synthetic lethality.
-
Targeting Glutamine Transporters
- Inhibiting transporters like SLC1A5 cuts off the glutamine supply, further restricting growth.
-
Immunometabolism and T Cell Activation
- Glutamine helps T cells during activation and affects their functions. Using glutamine-targeted therapies alongside immune treatments could enhance anti-cancer effects.
-
Biomarkers for Glutamine Metabolism
- Tracking glutamine-related markers can help personalize treatments.
-
Combination Approaches
- Combining glutaminase inhibitors with standard therapies may boost effectiveness. This strategy can also increase the sensitivity of cancer cells to certain drugs.
-
Balancing Treatment and Toxicity
- GLS inhibitors can selectively target cancer cells, minimizing harm to normal cells.
-
Diet and Glutamine Intake
- Reducing dietary glutamine might enhance therapy effects. However, the relationship between diet, metabolism, and treatment is complex.
-
Metastasis Prevention
- Targeting glutamine metabolism can lower the risk of cancer spreading by limiting the cells’ ability to adapt.
9. Clinical Progress of Glutaminase Inhibitors
Glutaminase inhibitors selectively target the overactive GLS in cancer cells, sparing GLS2 in normal cells:
- Blocking EMT (Epithelial-to-Mesenchymal Transition): These inhibitors can help prevent EMT, which supports cancer spread.
- Combining with Immune Therapies: Because glutamine fuels T cells, pairing inhibitors with immune treatments boosts anti-cancer effects.
10. Future Directions
- Screening for New Drugs: Automated techniques can help find better glutaminase inhibitors.
- Precision Medicine: Tailoring treatments based on tumor metabolism profiles can improve outcomes.
- Exploring Beyond Metabolism: Glutamine may affect gene regulation, offering new therapeutic possibilities.
11. Conclusion
Glutamine metabolism plays a crucial role in cancer cells and immune cells like T cells. It influences processes such as autophagy and ROS regulation, impacting cancer growth and survival. Targeting this pathway offers a promising strategy for cancer therapy, especially in combination with other treatments. As research progresses, more effective approaches will emerge.
AxisPharm provides comprehensive mass spec based metabolomics services, including untargeted and targeted metabolomics services.
Reference
[1]Altman B J, Stine Z E, Dang C V. From Krebs to Clinic: Glutamine Metabolism to Cancer Therapy[J]. Nature Reviews Cancer, 2016, 16(10):619.