Extracellular matrix-derived matrigel contains a variety of growth factors. And it has ECM properties that support cell proliferation, migration, and differentiation, so that widely apply in cell culture in vitro. However, this type of matrigel contains unknown components, has poor stability, and lacks controllable physical properties. Therefore, it is particularly important to develop a controllable biomaterial with both ECM function and reasonable design.
Professor Manuel Salmeron-Sanchez’s team from the University of Glasgow publish an article “Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors” in the Biomaterials journal. The researchers used polyethylene glycol to covalently bind the overall length of fibronectin, prepared a PEG-fibronectin hydrogel that can enrich and control growth factors. And proved that the hydrogel can combine intravascular growth factor(VEGF) and bone morphogenetic protein (BMP2) to promote angiogenesis and achieve bone regeneration.
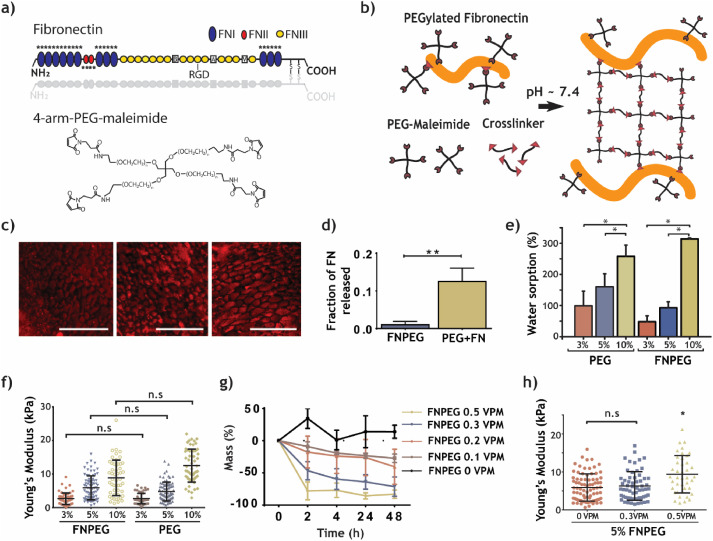
Preparation and characterization of FNPEG hydrogel
FN covalently binds to the PEG network through a Michael-type addition reaction to form an FN-labeled FNPEG hydrogel. FN immunofluorescence staining and release detection results show that FN is evenly distributed in the hydrogel network and the binding is stable. PEG can control the physical and chemical properties of the FNPEG hydrogel system. With the increase of the PEG content in the system, the swelling performance and Young’s modulus of the FNPEG hydrogel are also significantly improved. In addition, the addition of a 0.5VPM degradable crosslinking agent will enhance the mechanical strength of the hydrogel.
FNPEG hydrogel can stably bind growth factors
FN can randomly bind to growth factors, such as binding to VEGF through FNIII12-14. Therefore, compared with PEG, FNPEG hydrogel can effectively copolymerize a large number of growth factors. The researchers used fluorescently labeled VEGF to track its release over time. The fluorescently labeled VEGF was mixed into PEG and FNPEG hydrogels for release experiments. After 24 hours, PEG released all VEGF, and FNPEG hydrogels retain 50% of VEGF. At the same time, PEG and FNPEG hydrogels are placed in the same concentration of fluorescently labeled VEGF to test their ability to bind VEGF.
The results show that FNPEG hydrogel binds 2μg more VEGF than PEG per milliliter. In addition, the presence of the degradable cross-linking agent VPM will not affect the stable binding of the hydrogel to VEGF, indicating that the FNPEG hydrogel can control the release of growth factors through its own degradation properties.
FNPEG hydrogel can promote the formation of a microvascular network
There was no significant difference in the survival rate of HUVEC in PEG and FNPEG hydrogels. The researchers encapsulated the microcarriers inoculated with HUVEC into FNPEG hydrogel and Matrigel and added VEGF to the medium to study the effect of FNPEG hydrogel in promoting tube budding during 3D culture. In the FNPEG hydrogel with VEGF, a lot of blood vessels sprouted phenomenon similar to Matrigel appeared, and the area of connected blood vessel buds was more than that of Matrigel.
The researchers further encapsulated HUVEC and VEGF directly into FNPEG hydrogel to study whether FNPEG hydrogel can promote blood vessel formation in three-dimensional exchange like Matrigel. On the 1st and 2nd day, FNPEG hydrogel formed wider vascular clusters than the Matrigel group and PEG group containing VEGF. However, starting from day 3, the vascular clusters in the FNPEG hydrogel began to decompose, indicating These cell complexes are unstable. Pericytes, smooth muscle cells, and other types of cells are needed to stabilize the newly formed capillaries together.
The researchers used a more complex chicken embryo allantoic membrane to test whether FNPEG hydrogel can promote the formation of capillaries. The number of bifurcations and junctions of capillaries on the chicken embryo allantoic membrane of the FNPEG hydrogel with and without VEGF addition was significantly higher than that of the control group, indicating that FNPEG hydrogel not only has the ability to lasting continuously release VEGF, the VEGF produced by the chicken embryo allantoic membrane during the development process can also be retained in situ to promote capillary angiogenesis. However, there was no significant difference between the PEG hydrogel experimental group with and without VEGF and the control group. It was further verified that VEGF was released rapidly from PEG in the first few hours, resulting in a relatively rapid disappearance of VEGF from the local area.
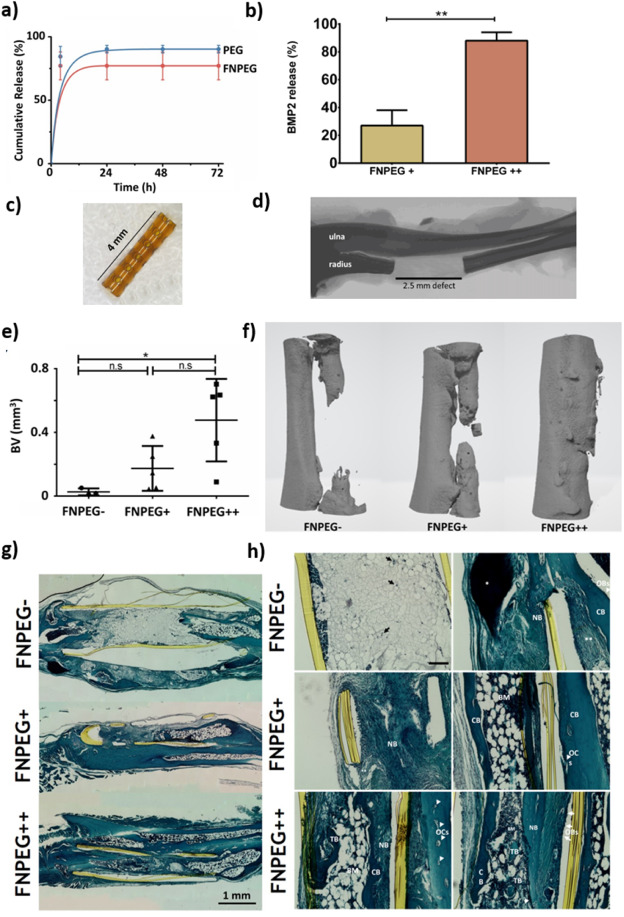
The load FNPEG hydrogel with BMP2 promotes bone growth
Researchers used adult mice to construct a nonunion (critical size) radius defect model and proved that FNPEG hydrogel loaded with BMP2 can promote bone growth and repair in vivo. The results of the bone defect repair of the hydrogel tubular scaffolds of each component showed that with the increase of BMP2, the bone growth from FNPEG- to FNPEG+ to FNPEG++ increased, and the FNPEG++ group achieved bone defect closure. The staining results of Safranine-O Fast Green and Hematoxylin tissue sections showed that there was a large amount of collagen deposition at the implantation of FNPEG+ and FNPEG++ hydro.
Reference
https://www.sciencedirect.com/science/article/pii/S0142961220303501?via%3Dihub