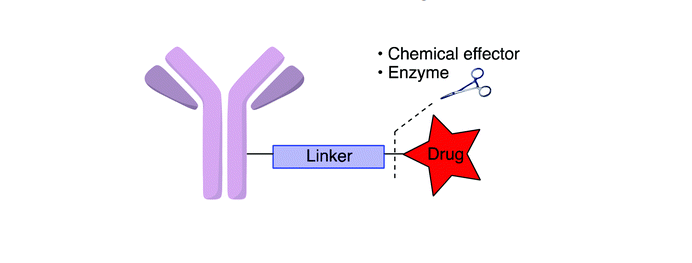
Antibody-drug conjugates (ADC) have been identified as the main treatment therapy for clinical cancer treatment. The principle of ADC is on the basis of monoclonal antibodies, which specifically direct toxic agents to diseased tissue. And minimizing peripheral damage to healthy tissues.
ADC involves a critical understanding of target antigen, conjugate internalization of tumor cells, drug potency, and stability of the linker with drug and antibody.
There is one of the main challenges in developing safe and effective antibody-drug conjugates is the generation of suitable chemical linkers between cytotoxic drugs and monoclonal antibodies. A stable ADC linker ensures that fewer cytotoxic payloads fall off before reaching tumor cells, increasing safety and limiting dose.
In the US FDA-approved antibody-drug conjugates currently, two common methods of linking cytotoxic anticancer agent payloads include cleavable linkers and non-cleavable linkers.
Cleavable linkers play a crucial role in the success of antibody-drug conjugates. They use the inherent properties of tumor cells to selectively release cytotoxins from ADCs. They are stable in the blood circulation for the long-term. The following three mechanisms are commonly used: 1.protease sensitivity; 2.pH sensitivity; 3.glutathione sensitivity.

Image resource: royalsociety.org
Recognition and cleavage of the specific peptide sequence in the linker utilize the “main protease” found in the lysosomes of tumor cells through a protease sensitivity strategy. Dubowchik and Firestone etc. al. pioneered the discovery of the valine-citrulline (VC) dipeptide as an intracellular cleavage mechanism by cathepsin B.
Compared to the cytoplasm(pH=7.4), the acid-sensitive strategy is to use a lower pH of the endosome (pH=5-6) and lysosome(pH=4.8) compartments to trigger the hydrolysis of an acid-labile group within the ADC linker. Such as hydrazone.
The third release strategy utilizes higher intracellular glutathione concentrations than in plasma. So that the disulfide-containing linker releases cytotoxins after reduction by glutathione.
Non-cleavable linker has no definite drug release mechanism. The ADC prepared by this strategy relies on the complete lysosomal proteolytic degradation of the antibody that releases the antibody-drug after internalization. Through this degradation, the non-cleavable linker carrying the drug will also be combined with the conjugated amino acid of the antibody. Therefore, ADCs with non-cleavable linkers are even more dependent upon the biology of the target cells than cleavable linkers.
It appears that for non-cleavable linkers, the advantage is increased plasma stability, and it can improve the therapeutic index. Studies showed the non-cleavable linked ADCs generally perform is better than cleavable counterparts in vivo.
Compared to cleavable linkers, due to the fact that payload derivatives from non-cleavable ADCs can kill target cells, non-cleavable linkers can potentially provide a larger therapeutic window. Finally, it is expected to reduce off-target toxicity compared to cleavable linker conjugates because non-cleavable ADCs can provide greater stability and better tolerated.
AxisPharm provides GMP standard PEG reagent to match customers’ quality requirements. PEG linkers, Click Chemistry Tools, ADC, bio labeling reagents, and more new products will be available by custom synthesis.

