After decades of development, ADC drugs have entered a mature stage, with 15 ADCs approved globally and several ADCs entering the frontline therapies, providing significant clinical benefits to cancer patients.
But at the same time, nearly 100 ADCs clinical pipelines have been terminated, primarily due to on-target/off-tumor toxicity and insufficient efficacy. Therefore, the development of the next generation of ADCs should start from the components of ADCs, comprehensively considering antibodies, ADC linkers, and payloads that match the characteristics of indications and targets to build safer and more effective ADC drugs.
01
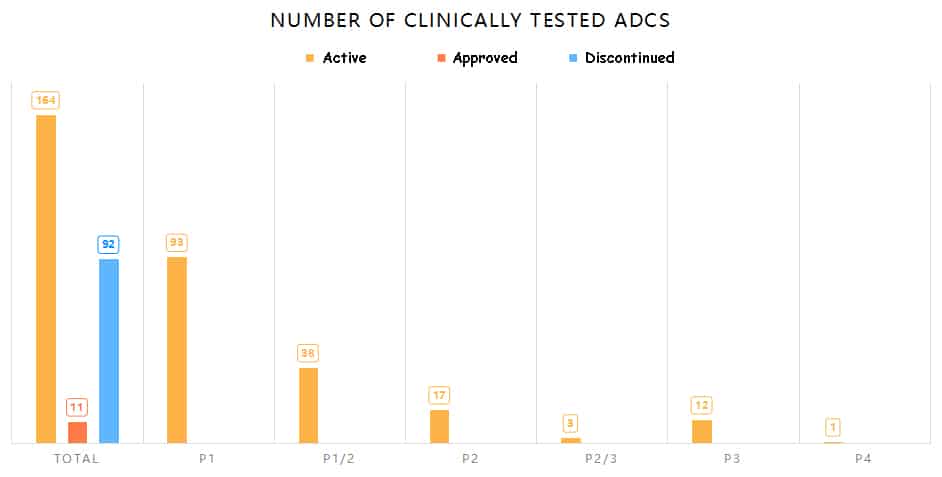
267 ADCs have entered clinical trials, among them, HER2 ADCs are the most.
According to statistics from Aarvik Therapeutics, since the first ADC drug “Mylotarg” entered clinical trials in 1997, there have been over 1200 anti-tumor clinical trials involving 267 ADCs in the past 26 years. Among them, 11 ADCs (excluding biosimilar ADCs and withdrawn Blenrep) have been approved by FDA, 92 ADCs have been discontinued, including 54 publicly announced terminations and 38 projects removed from the development pipeline by companies. Additionally, 164 ADCs are still in clinical development, with 32 of them in phase II and III trials.
FDA has approved a total of 11 ADCs involving 10 different tumor antigens, with 5 used for hematologic malignancies (CD19, CD79B, CD142, FRα, Nectin-4) and 5 for solid tumors (CD22, CD30, CD33, HER2, TROP-2).
On the other hand, the ADCs entering clinical trials involve a total of 106 different tumor antigens. Among these, HER2 ADCs has 41, which are the most, followed by Trop-2 ADCs, totaling 18. The remainder includes CLDN18.2 ADCs (11) and EGFR ADCs (11).

02
Over half of clinical ADCs utilize cleavable linkers
ADC linkers are mainly categorized into cleavable and non-cleavable linkers. Cleavable linkers may enhance bystander effects but could also lead to systemic side effects due to premature toxin release into the circulation. On the contrary, non-cleavable linkers, while reducing systemic toxicity, may weaken bystander effects, resulting in diminished efficacy.
Among ADCs entering clinical trials, 54% use cleavable linkers, while only 16% utilize non-cleavable linkers, and the remaining 30% have undisclosed linker information. Of the 11 FDA-approved ADCs, 10 employ protease-cleavable linkers, with Kadcyla being the only exception using a non-cleavable linker, reducing its systemic toxicity and off-target effects on HER2-low-expressing cells. In contrast, Enhertu, also targeting HER2, uses a cleavable linker. In a head-to-head clinical study between Enhertu and Kadcyla, Enhertu demonstrated significantly superior efficacy (mPFS 28.8 months vs. 6.8 months). This outcome is also influenced by the different toxins used; Enhertu employs the topoisomerase inhibitor Dxd, while Kadcyla uses the microtubule inhibitor DM1.
Related Reading about HER2:
HER2, the first ADC drug for HER2-positive gastric cancer, approved in Japan
Why Human epidermal growth factor receptor 2 (HER2) is an attractive target for ADC
The toxins/payloads used in these ADCs that entered clinical trials mainly consist of four types: microtubule inhibitors, DNA-damaging agents,topoisomerase inhibitors, and targeted small molecules. Among these, 57% of clinical ADCs utilize microtubule inhibitors, including marketed ones like Kadcyla and Elahere. The second most used are DNA-damaging agents, accounting for 17%. Only 7% of clinical ADCs use topoisomerase inhibitors, including marketed ones like Enhertu and Trodelvy. Additionally, in recent years, some ADCs have attempted to conjugate with targeted small molecule drugs, such as Bcl-xL inhibitors, TLR, and STING agonists.
The effectiveness of ADCs is influenced not only by the type of payload but also by the drug-to-antibody ratio (DAR), multidrug resistance efflux pumps on tumor cells, and the metabolic clearance rate of the payload. Therefore, for different tumor indications, appropriate linkers and payloads matching their characteristics should be selected to reduce the clinical failure rate of ADCs.

03
The main reasons for ADC development termination: insufficient efficacy and intolerable toxic effects
Reasons for the termination of ADC clinical development include poor safety/inefficacy/commercial factors, etc. According to Maecker H et al.’s statistics, approximately 29% of ADCs entered into clinical trials were terminated due to intolerable toxic effects, including ImmunoGen’s Bivatuzumab mertansine, targeting CD44v6, using the microtubule inhibitor DM1 as the payload. Nearly 80% of patients experienced skin toxicity, including erythema, localized blistering, and severe skin desquamation, leading to the termination of Phase I clinical trials in 2005. The side effects may stem from the on-target/off-tumor toxicity of Bivatuzumab mertansine, as CD44v6 is also expressed on normal proliferating epidermal cells. Pfizer’s TROP-2 ADC was terminated in Phase I clinical trials in 2016 due to the high toxicity of the selected payload, resulting in severe toxicity before reaching the therapeutic dose. Conversely, Sacituzumab Govitecan, targeting TROP-2 and using a less toxic topoisomerase inhibitor, was approved by the FDA in 2020.

In addition to intolerable toxicity, insufficient efficacy is also a major reason for terminating ADC development, with approximately 47% of all terminated ADCs stopped due to inadequate efficacy. Factors contributing to inadequate efficacy of ADCs include:
- Low density of tumor target antigens or poor internalization characteristics of ADCs;
- Insufficient toxicity of the payload;
- Suboptimal Drug-to-Antibody Ratio (DAR) of the ADC;
- Incomplete release of the payload outside the tumor or incomplete drug release within the tumor;
- Poor pharmacokinetic (PK) properties leading to rapid clearance of the ADC;
- Failure to demonstrate superiority over standard therapy;
- Increased levels of multidrug resistance mediated by drug efflux transporters in tumors.
NJH395, an immune-stimulating antibody drug conjugate developed by Novartis targeting HER2 with a TLR7/8 small molecule agonist, was terminated in Phase I clinical trials, possibly due to insufficient activity of the TLR7/8 small molecule agonist, as no objective responses were observed in the 18 patients enrolled in the trial.
CMB-401, developed by Pfizer, is an ADC targeting MUC1. It was terminated in Phase II clinical trials for NSCLC due to poor efficacy, believed to be caused by the premature release of the amide-conjugated payload, resulting in insufficient payload reaching the tumor.
Furthermore, seven ADCs, including rovalpituzumab tesirine (DLL3), depatuxizumab mafodotin (EGFRvIII), AMG 595 (EGFRvIII), AGS16F (ENPP3), glembatumumab vedotin (gpNMB), lifastuzumab vedotin (NaPi-2b), and lorvotuzumab mertansine (CD56), were terminated because they failed to demonstrate superior efficacy to existing standard treatments in clinical trials.
Overall, most ADCs fail due to excessive toxicity and insufficient efficacy. The toxicity mainly arises from the on-target toxicity of ADCs binding to antigens in normal tissues, while insufficient efficacy manifests despite reaching the maximum tolerated dose (MTD) of the payload but still lacking efficacy at the given dose.
04
ADC optimization needs to consider various demands such as targets, antibodies, linkers, and payloads comprehensively
While several ADC drugs have already received regulatory approval, the development of new generations with greater therapeutic indices, stronger therapeutic effects, and higher safety remains imperative.
Optimizing ADCs requires comprehensive design and consideration of each component, including target antigens, antibodies, linkers, conjugation methods, and payloads.
Regarding target antigens:
Attention should be paid to the biological characteristics and expression patterns of target proteins, which determine the choice of antibody binding affinity, whether high or low. Similarly, this affects the selection of payload potency and mechanism of action. Since tumor-specific antigens are generally limited, many clinically used ADCs target tumor-associated antigens that may also be expressed in normal tissues, necessitating the selection of payloads with lower toxicity. Additionally, excessively high antibody affinity should be avoided. Enhancing antibody binding specificity and internalization can also improve the success rate of ADC development. For example, the use of bispecific antibodies can enhance tumor-specific recognition and increase the efficiency of payload internalization.
Regarding linkers and conjugation methods:
They should be matched with the potency, solubility, metabolism, and mechanism of action of the payload. Over half of the approved ADCs use cleavable peptide linkers, with payloads randomly attached to cysteine or lysine residues on the antibody. Although site-specific conjugation methods have been developed, no related ADCs have been approved yet.
Data suggest that broad use of payloads such as auristatin and maytansinoid without considering target antigens or tumor biology makes it difficult to develop successful ADC drugs. Many ADC pipelines that mimic Adcetris (using auristatin as a payload) and Kadcyla (using maytansinoid as a payload) have failed as a result. Ideal ADC payloads should have sufficient cytotoxicity against tumor cells, moderate water solubility, short half-life, and bystander effects to enhance efficacy and reduce systemic toxicity.
ADC drugs combine small molecule toxins with antibodies, achieving targeted delivery of chemotherapy drugs. DS-8201, in particular, has brought considerable efficacy to many cancer patients. However, due to multiple factors such as target antigen expression in normal tissues, significant increase in payload toxicity, and instability of linkers, there is still room for optimization of current ADC drugs. Selecting appropriate target antigens and antibodies, and screening modified payloads and linkers matching the targets and indications, will help enhance the efficacy and specificity of ADCs, achieving potent therapeutic effects with minimal toxicity, thus benefiting more cancer patients.
References:
[1] Pharmacodia Database
[2] Maecker H, Jonnalagadda V, Bhakta S, et. al. Exploration of the antibody-drug conjugate clinical landscape. MAbs. 2023 Jan-Dec;15(1):2229101.
[3] Hurvitz SA, Hegg R, Chung WP et. al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023 Jan 14;401(10371):105-117.
[4] https://www.adcreview.com/
Related Reading:
